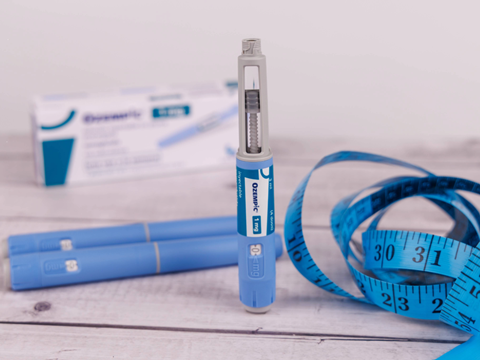
Weight-loss drugs keep gaining traction – is that a blessing or a curse for the packaging industry? We dive deeper into the implications of smaller portion sizes, pharmaceutical safety practices versus circular packaging initiatives, supply chain traceability, and more.
According to a recent poll, one in eight adults in the United States is using a GLP-1 drug, whether to treat a chronic condition or lose weight. The growing acceptance of peptides in weight management applications is exemplified by the World Health Organization releasing its first guideline on using GLP-1 therapies as a chronic obesity treatment.
Roland Berger also foresees a price drop as first-generation drugs come off patent and competing products are introduced. In short, GLP-1s are becoming ever more accessible, and the packaging industry is responding.
A report from Big Chalk predicts that the use of GLP-1s could lead to a 1.3% to 3.1% loss in food volume for groceries this year. Apparently, 28.2% of GLP-1 users in the United States have downsized the packets of crisps they purchase, and 26.4% are buying smaller soft drinks.
Economic factors, such as the aftershocks of post-pandemic inflation, could also play a role in this trend. Even so, the growing prevalence of GLP-1s has reportedly influenced the way consumers think about portion sizing, even if they aren’t taking the medications themselves.
“Retailers need to reassess private label, shelf allocation and online presence to cater to GLP-1 users,” Roland Berger asserts. “This includes promoting products that meet the needs of these consumers, such as smaller serving sizes and “GLP-friendly” items.”
Some supermarkets are starting to label their products as ‘GLP-1 friendly’, but MedPage Today cautions that these labels are not FDA-regulated and may not align with a GLP-1 user’s nutritional requirements. Instead, 3D Color recommends using phrases like ‘high protein’, ‘low added sugar’, ‘good source of fibre’, and even exact gram measurements of the relevant food groups.
Another consideration is whether shifts in consumers’ appetite will lead to adjustments in portion sizing. Research from Circana already indicates that the food industry will feel different impacts per food group, with GLP-1 users expected to eat more produce and deli foods, but fewer frozen goods, within their first year on the drug.
Companies like Nestlé have already released portion-aligned product lines, which require existing packaging formats to be downsized. Alternatively, multi-serve products can transition into releasable formats through zip closures or snap lids – accommodating less hungry consumers by keeping the product fresh between portions.
Meanwhile, the pharmaceutical industry must consider the requirements of the drugs themselves. Injectables must be stored at temperatures of 2–8°C, even in transit – and as Pelton Shepherd points out, a shipping container will leak cold air if it contains dead space. This must be filled with inserts and cushioning, or else the box must be downsized appropriately.
Companies are starting to balance these requirements with sustainability considerations. For example, Nordic Cold Chain Solutions offers its Ice-Eco Gel Packs, which comprise a kerbside recyclable paper pouch and an ASTM 6400-certified compostable gel interior. However, any remaining dead space must be filled with inserts, cushioning, or else a downsized box.
Woolcool also manufactures thermal insulation from pure wool, as well micro-perforated MDPE for moisture control. Apparently, the solution cuts down on carbon during this lifespan and is compostable at end-of-life.
Transporting medication during use is another consideration. Specific storage instructions vary between brands and formats, but consumers can keep their treatments cold with portable coolers or insulated bags, widely available from retailers like Amazon.
These containers can be reused, but GLP-1s generate single-use packaging waste in other ways. Users are sometimes encouraged to disinfect their skin before injecting, which may lead them to carry alcohol wipes – many of which are packed in flexible plastic.
Used needles also require puncture-proof sharps bins for safe disposal. Generally made from rigid polymers, the bins must enter the clinical waste stream, either through dedicated collection services or by returning the container to a pharmacist or general practitioner.
Traditionally, the whole bin is incinerated for safety reasons. More recent efforts have sought to separate the waste from the container; for example, the UK’s National Health Service has collaborated with waste management company Stericycle to sanitize and reuse tens of thousands of sharps bins.
Shipments must also be tracked and serialized for fulfilment and visibility purposes. Digital temperature loggers are recommended to signal temperature changes and keep products cool at every stage of the supply chain.
To name one example, Nordic Supply Chain has designed its Thermis Tag 1E temperature indicator with a default alarm range of 2–8°C for GLP-1 medications. Said to monitor the temperature of a pack for up to 400 days, it is recommended for high-volume shipments and local pharmacy deliveries alike – but, notably, the tags are single-use.
As is the case across the pharmaceutical industry, traceability also combats the threat of counterfeit products or tampering. Organizations like the FDA and European Medicines Agency have raised the alarm about drugs falsely marketed as GLP-1 products, which raise serious health concerns.
As rules and regulations around GLP-1s continue to develop, McAfee urges consumers to gauge a product’s reliability via its packaging. Signifiers include visibly tampered or relabelled boxes, labels and instructions that do not appear in the consumer’s native language, and/or discrepancies in – even a complete absence of – lot and serial numbers.














No comments yet