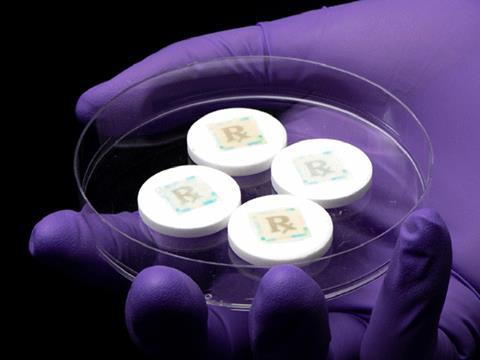
As fake medications and pharmaceutical products proliferate across online pharmacies, which can be easily accessed via smartphone. a new anti-counterfeiting technology can turn the phone into a potentially lifesaving security device by simply taking a picture of a cyber-physical watermark to confirm if the medication is real or not.
The technology has been developed by a team of biomedical engineers from Purdue University in the USA, and the study has been published in the Advanced Functional Materials journal. Young Kim, associate head for research and an associate professor at Purdue’s Weldon School of Biomedical Engineering, says the continued rise of counterfeit medications, pharmaceutical products and medical supplies can be attributed largely to the increase of online pharmacies, many of which are unregulated.
“We have technology that can empower patients to check to see if the cyber-physical watermark on the medications they are taking is real or counterfeit,” Kim said. “This allows them to also confirm dose, frequency and information on the medicine.”
The watermark is printed on specialized fluorescent silk with FDA-approved food dye through an inkjet printer – a common technique that bakers use for placing edible photos on cakes. The silk is all protein and can be edible, say the researchers.
“Silk is a great choice for eating, as we also were not wanting to use synthetic or artificial materials and fluorescent silk makes it very difficult to duplicate the watermark.” Jung Woo Leem, a postdoctoral research associate, added that the silk is a good option for pharmaceuticals because engineers have the ability to change the biopolymer’s shape, structure and flexibility.
“We are affixing a watermark on an individual medicine that is readable by a smartphone camera to extract a hidden digital key,” Kim continued. “Purdue has an excellent track record of watermarking and inkjet printing research.” The engineers also addressed how to use the technology with different smartphone models, photo quality and light.
To address the growing counterfeit issue, the US Food and Drug Administration is requiring that medications have unit-level traceability by 2023 through the Drug Supply Chain Security Act. Kim said, while pharmaceutical companies have the ability to track boxes or sheets of medications, adding traceability directly on a pill could require adding numerous manufacturing and data management steps.
Along with the anti-counterfeit applications, the on-dose printing method could also aid the use of single-unit or unit-dose packages in hospital pharmacies which could “lower the risk of dispensing errors, improve inventory tracking, enhance security, and minimise labour costs,” they claim.
Placed on pills using a simple sugar glue, the smallest size of watermark the team could produce is 5 X 5 mm. While the team has had success with solid pills, it is working also on developing technology for liquids. Kim said that the technology could be used first on name-brand medications and restricted narcotics before being rolled out on over-the-counter medications and generics.














No comments yet