Aptar CSP Technologies, a part of AptarGroup, Inc., has announced the launch of a “first-of-its-kind” active packaging product. The new technology utilizes the company’s patented 3-phase Activ-Polymer platform within its Activ-Film product configuration.
Aptar’s new product builds on the recent U.S. FDA approval of an HIV prevention medicine expected to launch in Q1 2020 that utilizes the company’s Activ-Blister packaging solution to provide moisture adsorption and drug product stability.
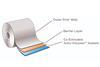
Aptar CSP Technologies added the combined oxygen scavenging and moisture adsorption capability to it’s Activ-Film product to provide customers with a fully integrated solution for drug products that are sensitive to both moisture and oxidation. According to the company, this unique proprietary formulation provides unparalleled dual protection in various packaging configurations, such as Activ-Blister, and broad application fields ranging from oral solid dose and transdermal to diagnostics and probiotics.
“This is an important milestone for Aptar CSP as we continue to expand the capabilities of our 3-phase Activ-Polymer platform technology to accelerate and de-risk the product development process, providing solutions that improve and save patients’ lives,” said Badre Hammond, Vice President Commercial Operations, Aptar CSP Technologies. “This technology can help reduce packaging complexity while providing a custom solution to stability concerns, enabling pharmaceutical companies to enhance drug stability and support speed-to-market.”
Aptar CSP Technologies’ 3-phase Activ-Polymer platform technology offers customized active packaging solutions designed to meet the drug developer’s specific formulation as well as provide a broad spectrum of drug-specific protection, including aroma emitting, volatile organic compounds (VOCs) scavenging, and anti-microbial scavenging.