In an ever-tightening regulatory landscape, pharmaceutical and biopharmaceutical manufacturers are always looking for ways to improve the safety and efficiency of their drug supply chains. An important part of that process is the fill/finish step, where drug products are placed into vials, stoppered, and, traditionally, capped with aluminum seals and crimped.
Sylvia Marzotko, Senior Manager, Global Product Management, West Pharmaceutical Services, Inc., and Piotr K. Wagner, Senior Specialist, Product Management Daikyo Portfolio, West Pharmaceutical Services Deutschland GmbH & Co KG, explore the value of plastic caps in drug supply chains.
In recent years, drug delivery manufacturers have begun to develop alternative components that can improve safety and potentially avoid expensive errors that can occur during the fill/finish process. Enter plastic caps. Plastic caps present a potentially unique value for pharmaceutical R&D projects and drug fill/finish operations by eliminating some steps from conventional fill/finish lines. Eliminating these steps not only makes the process more efficient, but also can make it safer by reducing certain risks.
Traditionally, there are four stations throughout conventional fill/finish lines:
1) Filling station, where the drug product is placed into the glass vial
2) Stoppering station, where a stopper is placed on the vial
3) Capping station, where an aluminium seal is placed over the vial
4) Crimping station, where the aluminium seal is crimped, creating the final, integral seal
In most cases, vials undergo this process one-by-one, which can take a long time, depending on the size of the batch. Also, when dealing with aseptic environments, each additional step presents another chance to introduce risk into the process. Along the way, problems can occur, such as aluminium particulates appearing around the rim of the vial during the mechanical crimping process, or overcrimping, which can lead to glass breakage. Any of these issues can be incredibly costly for pharmaceutical manufacturers, who may need to reject an entire batch if even a small number of vials were not properly sealed.
Plastic caps can help to avoid some of those risks by completely eliminating the need for stoppering, aluminium capping and crimping. They can do this because they are manufactured with a preassembled stopper in place. And, because some versions are available in a nested format, the entire nest of vials with up to 100 pieces can be closed simultaneously, saving valuable time and making the supply chain safer and more efficient.

Sylvia Marzotko, Senior Manager, Global Product Management, and Piotr K. Wagner, Senior Specialist, Product Management Daikyo Portfolio
Plastic caps and lyophilized drug products
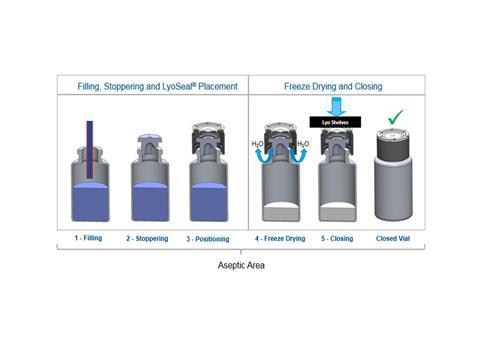
Another area in which plastic caps can potentially add value is in the filling process of drugs that need to go through lyophilization. These drug products are usually highly unstable, and are placed through the process of lyophilization to remove the water, allowing them to remain stable for longer. But the process is time-consuming, extremely expensive and introduces even more areas for potential risk.
Traditionally, once the drug product is placed into the vial and inserted into the lyophilization chamber, it undergoes a process of being frozen, then a vacuum is created, followed by sublimation, where the water is removed. A shelving apparatus then lowers, placing a rubber stopper in the entire batch. While in the lyophilization chamber, a number of problems can occur, including stopper pop up, loss of vacuum and the stopper sticking due to the extremely cold environment, which can result in product losses.
After this process, the stoppered drug vial needs to be removed from the chamber, at which point an aluminium seal is added and then crimped. It is this last step that is the most complicated and fraught with potential error, partially because of increasing regulatory requirements. For example, regulations from the European Commission such as EMA Annex 1, which states that lyophilized drugs must be capped and crimped almost immediately after exiting the lyophilization chamber. In order to reduce the time gap between lyophilization and crimping, during which product losses can occur that lead to rejects or noncompliance with regulatory standards, pharmaceutical manufacturers are looking for innovative capping solutions. As the need for lyophilized drug products exists and continues to grow, creating solutions to improve the processes and reduce the risks associated with these challenges has become increasingly important.
Plastic caps can revolutionise this process. They provide an instant sealing solution for lyophilized drug products all while inside the lyo chamber. Minimising the risk associated with established lyophilization processes, while maintaining the necessary sealing requirements.
The plastic cap is placed on the partially stoppered vial prior to entering the lyophilization chamber by the well-established “pick and place” method that incorporates the use of a feeder bowl and feed tracks. Once the cycle is complete, the collapsing shelves simultaneously fully insert the stopper into the vial and secure the cap to the vial crown, immediately protecting the product in the vial. The closed vial can proceed to final drug product inspection, completely bypassing the crimping station and thus streamlining the fill-finish process. This approach ensures that the fill/finish process adheres to regulatory requirements because the sealing takes place within the aseptic environment of the lyophilization chamber.
In both the case of non-lyophilized and lyophilized drugs, using plastic seals also offers the benefit of limiting the amount of physical space needed on fill/finish line environments because the capping station can essentially be removed. This can allow for this space to be reimagined and perhaps offer new ideas about how to use the space to keep the supply chain as efficient as possible.
Reacting to evolving needs
Plastic caps for non-lyophilized drug products show their advantages in flexible fill/finish lines that utilise robotic processes in which nested vials are matched with a nested and pre-assembled closure format. For these reasons, plastic caps work well with smaller batches of drugs products on a line that allows for flexible switchovers. But plastic cap solutions are expanding beyond the nested formats. For lyophilized applications, plastic caps in bulk format provide an immediate capping solution within the lyophilization chamber. They simplify vial closure and are therefore also suitable for manual crimping in early phases of drug R&D.
Tightening regulatory guidelines and the risks involved with the lyophilization and crimping process have pharmaceutical and biopharmaceutical manufacturers looking more and more into solutions that can be provided in bulk and nested configurations in order to keep them compliant.
Looking forward
As regulatory guidelines continue to tighten globally, pharmaceutical manufacturers will seek out innovative solutions to capping that allow them to remain compliant. While it will still take some time for plastic caps to provide a widespread solution for capping, they are already operational in certain fill/finish environments. In these environments, they are adding value in certain situations and have the potential to open up increased efficiencies in drug supply chains by eliminating steps from the fill/finish process, thereby reducing risk and likely creating additional space in fill/finish environments.
Reducing risk while creating a more efficient supply chain are two areas that are appealing to any pharmaceutical manufacturer. The end goal is making sure the drug product reaches the end-users, patients, where it has the ability to have a positive impact on their lives.















