
In the latest edition of the Spotlight, OPTEL tells us about its CartonPro system for pharmaceutical packaging lines, aiming to provide flexibility and optimize operational throughput for manufacturers.
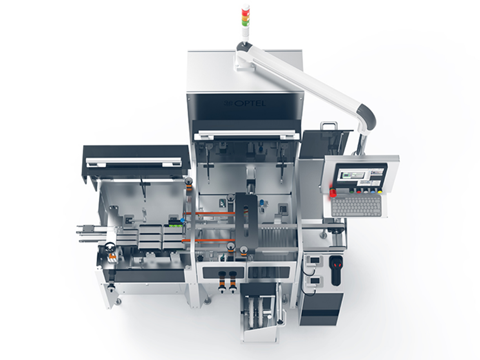
In an industry where precision, compliance and efficiency are essential, CartonPro™ delivers exceptional value. Specifically designed for pharmaceutical packaging lines, this all-in-one station from OPTEL integrates print and inspect, tamper evident and checkweighing functionalities into one compact, high-performance system - helping manufacturers meet global compliance standards while optimizing operational throughput.
Built for flexibility, reliability, and compliance, CartonPro™ fits easily into existing layouts with its compact footprint. Its front-facing design and intuitive touchscreen interface simplify operator interaction and maintenance. Whether launching a new line or upgrading for compliance, CartonPro™ helps manufacturers do more with less space, downtime, and hardware.
Compliance without compromise
Engineered for speeds up to 400 products per minute, CartonPro™ prints, inspects, weighs, and seals every unit with precision. The system features a servo-driven conveyor for smooth product handling across a range of carton sizes. High-resolution vision systems verify print and serialization data in real time, while its tamper-evident module applies dual-sided seals and verifies placement using glare sensors - boosting packaging integrity and consumer safety.
The integrated checkweighing system confirms every carton meets defined weight thresholds, using high-precision weigh cells and pneumatic ejection to remove non-compliant items before they progress downstream. This built-in quality control helps reduce product giveaway and recall risk while enhancing overall accountability.

Modularity and integration that scale with you
CartonPro™ stands out for its modular architecture, enabling fast, tool-free changeovers to accommodate box sizes, label formats, or production shifts with minimal disruption.
With full API integration, CartonPro™ connects seamlessly to existing MES and ERP environments. It ensures robust serialization, inspection, and compliance tracking—supporting real-time traceability and audit readiness across your packaging process.
Built on proven expertise
With more than 35 years of experience in pharmaceutical serialization, OPTEL designed CartonPro™ as a purpose-built solution for the industry’s evolving compliance and efficiency needs. From built-in alignment with EU FMD and DSCSA regulations to turnkey installation support, the system simplifies complex packaging workflows into one streamlined solution.
Its stainless steel construction ensures long-term durability in high-demand production environments, while intuitive controls and simplified validation processes make it a smart investment for sustainable operations.
One system, ready for every change
CartonPro™ is ideal for packaging engineers, automation managers, and QA leaders seeking to upgrade or scale packaging lines with confidence. Designed for reliability, scalability, and smart integration, CartonPro™ provides the operational intelligence and regulatory assurance today’s pharmaceutical teams demand.
To discover how CartonPro™ can transform your packaging operations, visit www.optelgroup.com or contact us.
CartonPro™ — built for compliance, engineered for reliability.
This content was sponsored by OPTEL.









