Researchers led by Chiba University have utilized coformer molecules to improve the solubility of supramolecular polymers and, it is hoped, result in recyclable and biodegradable plastic materials in the future.
Supramolecular polymers, or SPs, are made of small molecules that are non-covalently bonded – i.e., no chemical reaction has taken place – in small repeating units. They are designed for improved stability, with variations in monomer concentration, solvent composition, and temperature enabling the creation of versatile, self-assembled structures in a process known as polymorphism.
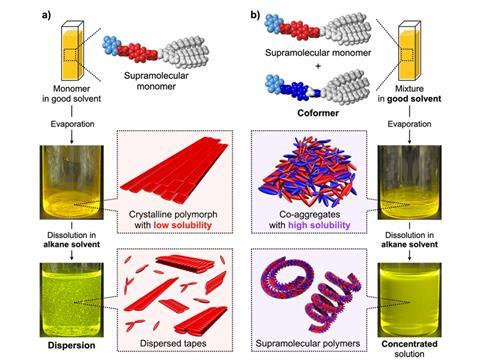
Whereas covalent or chemically bonded polymers are non-biodegradable, SPs are envisioned for use in ‘highly recyclable’ polymer materials – yet this application is restricted by the low solubility of monomer compounds in a crystalline polymorphic state.
On the other hand, a ‘coformer’ molecule is used to prevent crystalline polymorphic states from forming, co-aggregating with the parent molecule to improve solubility. A research team led by Professor Shiki Yagai from the Institute of Advanced Academic Research at Chiba University has expanded on this concept by applying a supramolecular monomer and forming an insoluble crystalline polymorphic state.
The paper has been published in Angewandte Chemie International Edition and is co-authored by Atsushi Isobe from Chiba University’s Graduate School of Science and Engineering and Dr. Takashi Kajitani from the Open Facility Center at the Tokyo Institute of Technology.
To overcome the poor solubility of previous SPs, the newly designed compound comes with a slightly different molecular structure as a supramolecular coformer. When mixed in with what the team describes as ‘a π-conjugated monomer functionalized with a barbituric acid multiple hydrogen-bonding unit’, a previous form of SP, the new compound is said to enhance the solubility of the parent monomer without impacting the ability to form a supramolecular polymer.
This hopes to overcome previous roadblocks surrounding the preparation of SPs at high concentrations and, as the monomer bindings are reversible, enable the separation of coformer molecules from the resulting SPs. Therefore, it is hoped that more recyclable and biodegradable plastics can be developed through this process in the future.
“Use of coformer can facilitate the large-scale production of SPs, leading to the evaluation of their functionalities,” explains Dr. Yagai. “The application of supramolecular polymers can contribute to the production of novel plastic materials showing high recyclability originating from the reversibility of monomer bindings and recycle them with lower energy consumption.”
In the further pursuit of sustainable plastics on a molecular level, researchers at the University of Washington have created new bioplastics thought to degrade at the same rate as a banana peel in a home compost bin, and scientists at the University of Konstanz have revealed a ‘self-healing’ mineral plastic that can be formed and reformed in water, now enhanced with bio-based building blocks to break down in the natural environment.
Scientific journal ACS Catalysis has also declared Carbios’ PET-degrading enzyme for depolymerization processes the best-performing under industrial conditions.
If you liked this article, you might also enjoy:
The L’Oréal approach to packaging sustainability
The way we talk about plastic needs to change – here’s how to get it right
What steps is Apple taking to make its packaging more sustainable?














No comments yet