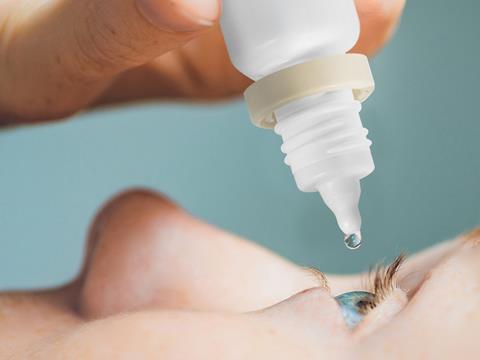
An enhanced design for Berry Healthcare’s established Rispharm R2 multidose eye dropper seeks to provide an improved patient experience in terms of safety and convenience, while maintaining its drug protection and delivery performance without changing pharma companies’ existing filling lines.
The new Rispharm R2 Multidose eye dropper meets FDA recommendations to ensure that the tamper-evident ring for any eye dropper stays connected to the bottle after opening with no risk of it falling off into the patient’s eye during administration of the product.
Berry Healthcare’s technical team has redesigned the tamper-evident band to provide a secure ring retention feature, while at the same time improving the container closure integrity when in use by the patient.
This further reduces the risk of product leakage and also optimises the opening and closing torque with the aim of enhancing convenience and ease of access for the end-user.
Berry says that for patients, these improvements have been achieved without any major changes to the design of the bottle, enabling them to maintain the same drug administration technique.
The company also says that its Rispharm R2 multidose eye dropper design offers enhanced convenience over single unit dose droppers, especially if the drug needs to be carried and administered away from the home, where a single bottle is easier and less bulky than boxes of single doses.
In addition, unlike single doses which should be discarded after opening, the multidose bottle allows all the product to be used, while the design of the Risdrop dropper reportedly ensures accurate dispensing of the drug, both of which avoid unnecessary waste.
The mono-material construction, which can be specified in both PE (polyethylene) and PP (polypropylene), means the full system can be recycled as one.
Rispharm R2 can also be produced with Advanced Recycling Material rHDPE and rPP. Meanwhile, Berry’s Bangalore India facility is now ISCC Plus accredited and can produce and deliver Rispharm R2 with such recycled materials. The Offranville, France facility, which also produces Rispharm R2, will be ISCC Plus accredited in 2021.














