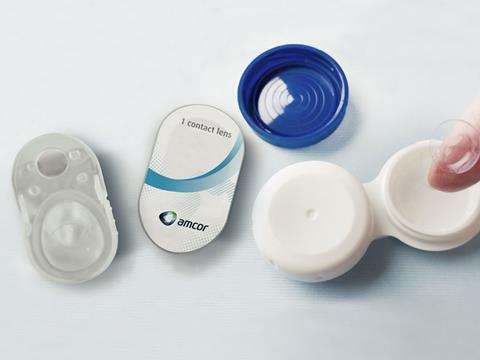
Amcor today announced the launch of a proprietary healthcare lidding technology that will be utilized for combination products – those consisting of two or more regulated components (device, drug or biologic).
This latest innovation from Amcor is based on a patented inert film development and laminate design. It provides a lidding solution that can withstand heat sterilization, the process of preserving and sterilizing items, while preventing drug uptake into the packaging.
According to the company, this new solution is suitable for combination healthcare products, such as devices with an Active Pharmaceutical Ingredient (API) that forms the basis of a medicine. Amcor says that it ensures machinability, integrity after sterilization, as well as a convenient peel opening for patients.
The features of the new product seek to complement Amcor’s existing healthcare portfolio, which includes solutions ranging from lidding for demanding sterilization environments, to high barrier overwraps protecting eye droppers and medications for the eye.
Amcor collaborated with Johnson & Johnson Vision over the course of several years to develop the new lidding technology. Each company apparently contributed specific skills and perspective, notably Amcor’s expertise with film extrusion, lamination and conversion for healthcare, and J&J Vision’s expertise on ophthalmic device packaging requirements.
Peter Konieczny, Amcor’s chief commercial officer, said: “We are bringing together industry-leading innovation and close customer relationships to develop the packaging solutions of the future. With this next-generation healthcare lidding technology we are opening a world of possibilities for products using active pharma ingredients. We look forward to extending this differentiated lidding technology to additional combination products in the future.”