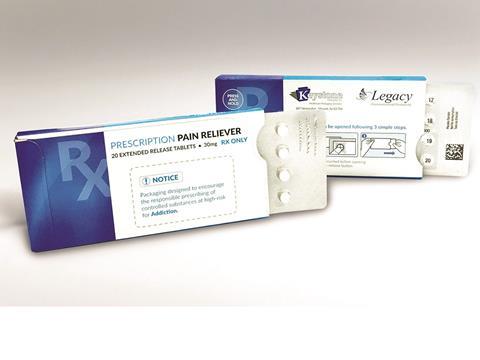
Keystone Folding Box Co., a designer and cGMP manufacturer of paperboard packaging solutions, has developed a modified, customisable version of its Ecoslide-RX prescription blister package to address the ongoing opioid epidemic in the US. The updated Ecoslide-RX package is designed to limit dosages in an effort to curb overdoses while providing added benefits for safe dispensing.
The lower dose count Ecoslide-RX was introduced in response to efforts by the FDA and the Institute for Safe Medication Practices (ISMP) to implement product packaging changes that deter opioid abuse. In an effort to combat dependency and overdoses, the design of the Ecoslide-RX allows doctors to prescribe smaller quantities of opioid medication via limited days of dosing presented in blister packs. The packaging also provides numerical identification of each dose, eliminating the possibility of pill-count errors.
Major benefits of Keystone’s original Ecoslide-RX package also are incorporated. The pack includes a re-closable locking feature offering consumers a safe yet streamlined experience. Child-resistant (F=1) yet senior-friendly, Ecoslide-RX is also versatile by design, meaning it can be adjusted to accommodate patients with dexterity issues. Additionally, the blister pack’s broad format allows for pictographs and larger texts on all sides for easier patient instruction and accessibility.
Per its name, Ecoslide-RX also is eco-friendly: Comprised of 100 per cent recyclable paperboard, the packaging separates easily from its internal blister for easy recycling.
“Ecoslide-RX can revolutionise the manner in which controlled substances are dispensed,” said Ward Smith, Director of Marketing & Business Development at Keystone. “It represents a forward-thinking solution that supports the pharmaceutical industry’s goal of combatting our opioid epidemic.”














