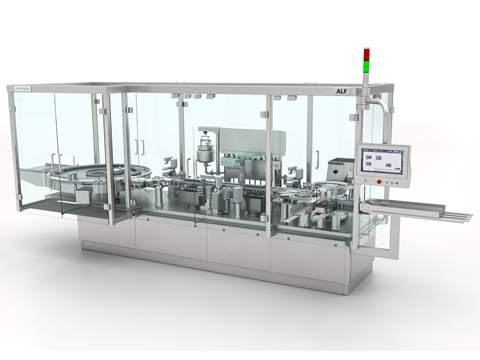
Syntegon has introduced its Settle Plate changer (SPC) at Achema 2024 in Germany, designed for automated viable monitoring in the aseptic filling process.
The company aims to support compliance with the latest Annex 1 for new and existing equipment. It says settle plates may be exposed to cleanroom air for a maximum of four hours and must then be replaced to ensure consistent sampling for liquid filling operations.
Apparently, with the new robotic handling unit this step can now be performed automatically, significantly reducing the previously required production interruptions. Syntegon also hopes to increase process reliability and traceability though optional barcode scanning.
The SPC is said to fulfill another important Annex 1 requirement: reducing manual operator intervention in the process zone for environmental monitoring by 80%, thereby minimizing the risk of contamination. In the second chapter of Annex 1, automation and robotic systems are emphasized under the keyword “appropriate technologies” for maintaining sterility.
The company states the SPC is available with the purchase of a new machine and as a retrofit for existing equipment. It can be integrated into “all machines and control systems from third-party suppliers”, apparently resulting in increased service life of existing equipment and reduced downtimes for pharmaceutical manufacturers.
“Environmental monitoring is essential in aseptic manufacturing and has become even more important in the context of EU GMP Annex 1,” says Steffen Gröber, global product manager service at Syntegon. “Thanks to the SPC, machines only need to be stopped once a day for viable monitoring, which results in noticeably higher machine availability of up to 300 hours per year and therefore more sustainable processes.”
Syntegon plans to present the SPC at CPHI Milan, Pack Expo Chicago and PMEC India.
In similar news, LeadEdge Flexo has released a new mounting material made of biobased polyethylene, seeking to lower reliance on synthetic virgin material in corrugated post-printing solutions. The material, known as cbak evolve, is a closed-cell foam mounting material said to contain at least 30% bio-based, third-party certified C14 material.
More recently, the company launched its 347 Plate Sealer, developed to improve additional production efficiencies. Forming a skin in 3 minutes and 47 seconds, the company says the 347 Plate Sealer has been formulated in response to customer feedback for a faster drying solution.
If you liked this story, you might also enjoy:
How are the top brands progressing on packaging sustainability?
The ultimate guide to global plastic sustainability regulation














No comments yet